Boostrix Injection In Pregnancy
If you are pregnant your name may be listed on a pregnancy registry.
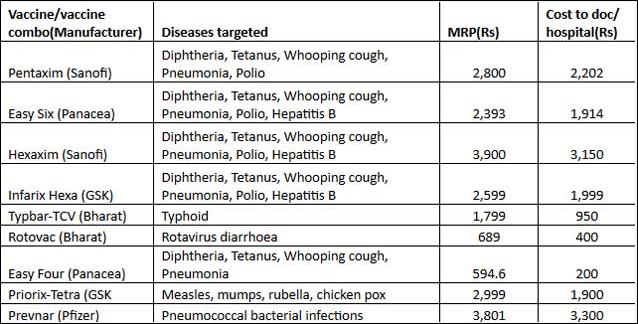
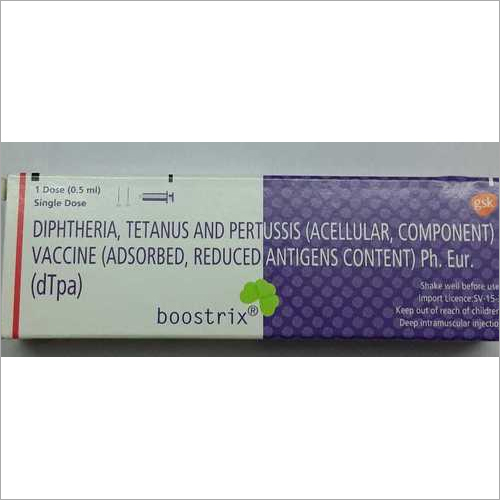
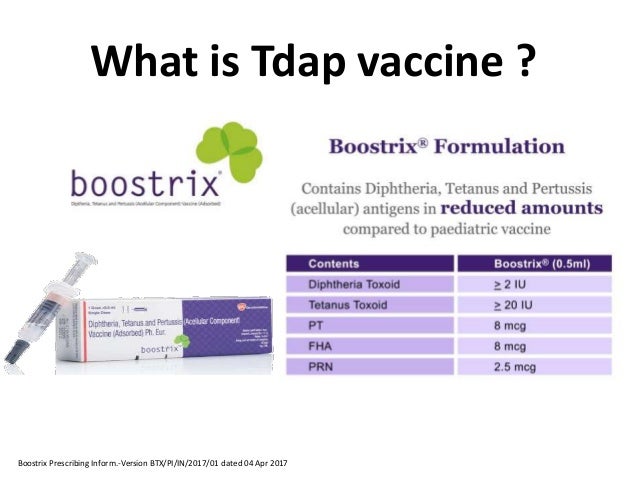
Boostrix injection in pregnancy. The vaccine does not contain any live bacteria or viruses and cannot cause any of the diseases it protects against. Both manufacturers of tdap vaccine sanofi pasteur for adacel and glaxosmithkline for boostrix created pregnancy registries to collect information from pregnant women who got tdap vaccine. Boostrix similar to boostrix ipv but without the polio component is one of the vaccines routinely recommended in the us for immunisation of pregnant women. The vaccine offered to pregnant women in the uk is called boostrix ipv.
Your doctor should determine whether you need boostrix during pregnancy. Safety history for tetanus and diphtheria vaccines. Boostrix isnt known to have harmful effects when you get the vaccine during pregnancy. However you may need boostrix vaccine during pregnancy to protect your newborn baby from pertussis.
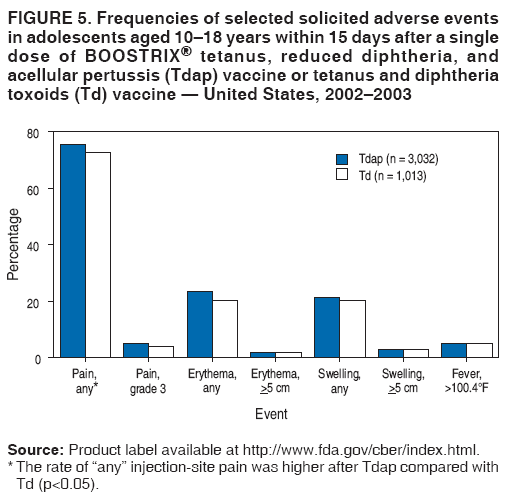
There have been no reported safety concerns in the us with the use of the vaccine in pregnancy. Boostrix is administered as a single 05 ml intramuscular injection into the deltoid muscle of the upper arm. Studies from the us and uk involving more than 40000 pregnant women found only mild side effects such as pain or redness in the arm where the vaccination was given. Boostrix is a vaccine that helps protect infants against whooping cough diphtheria and tetanus life threatening diseases caused by bacterial infections.
Yes the vaccine is safe for both the pregnant woman and baby when given during pregnancy. The manufacturers have not reported any safety signals to fda. There are no data to support repeat administration of boostrix. In fact the centers for disease control and prevention cdc recommends that all pregnant women get a tdap.
1 2 boostrix is funded for all nz children at 11 years of age as well as pregnant women. Allow time for the womans immune system to produce antibody protection against pertussis whooping cough reduce the risk that she will.








































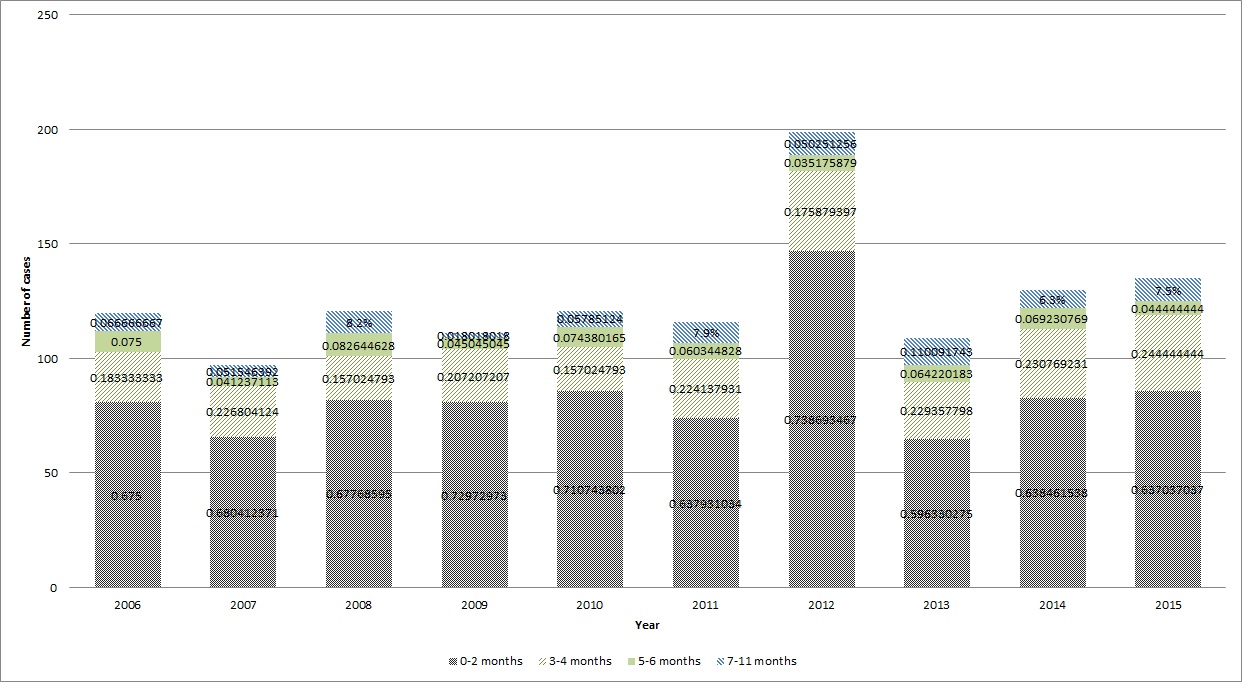

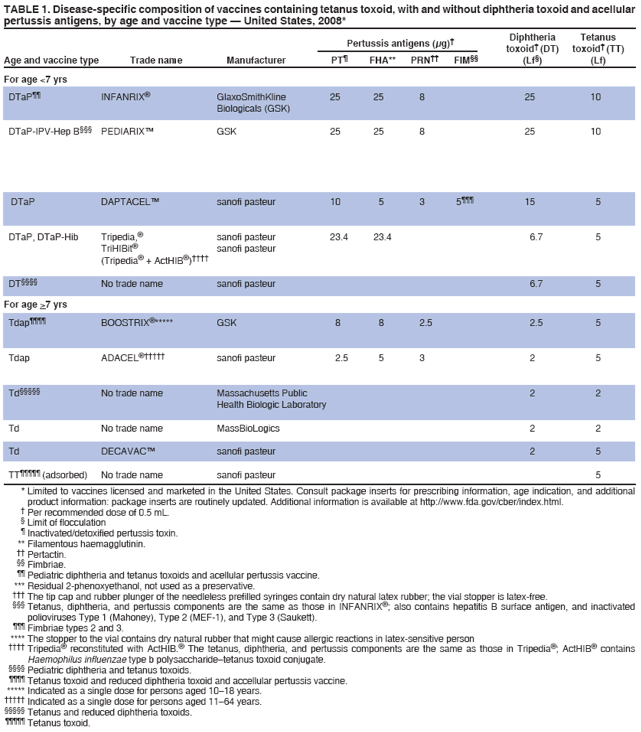






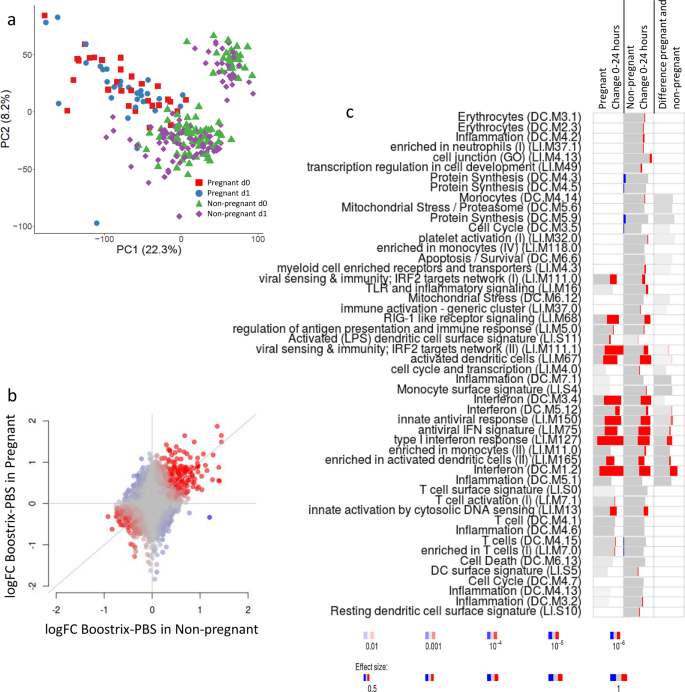






/doctor-giving-patient-injection-in-volunteer-clinic-573103329-5b3a6ad2c9e77c001a4b70d5.jpg)